Iron is the most abundant metal used in water distribution. Its chemistry is dominated by oxidation-reduction reactions among three valence states: 0, +2, and +3. Metallic iron is not stable in the presence of water (see Iron Chemistry), and is subject to corrosion by oxidation, probably microbially-mediated. A large variety of ferric and ferrous iron minerals are known, but only a handful are normally present in the corrosion scales of distribution systems.
 |
 |
|---|
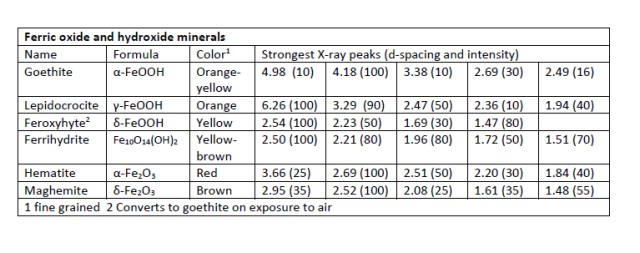
Ferric hydroxides and oxides
Ferric Hydroxide - Fe(OH)3. This substance forms if iron is precipitated rapidly, as in iron removal systems for groundwater wells. It is amorphous and so not a mineral. It will convert on aging to one of the more stable oxy-hydroxides.
Ferric Oxy-Hydroxide - FeOOH. Three different minerals have this composition: goethite, lepidocrocite, both of which are common in distribution systems, and feroxyhyte, which is found in deep-sea manganese nodules. The surface of these minerals has a high affinity for arsenic, lead, and other toxic substances, so they can be used in water treatment or, conversely, can act as transport agents for exposure to toxics.
Ferric Oxides - Fe2O3. Two minerals, hematite and maghemite have this composition. Both are common in nature, hematite much more so, but are seldom reported from distribution systems, possibly because they are too slow to form.
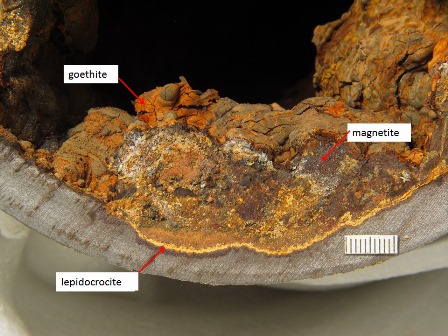
 Goethite in core of tubercle in cast iron water main, utility 02.Relicts of former hard shell magnetite and surface layer lepidocrocite mark earlier growth positions
Goethite in core of tubercle in cast iron water main, utility 02.Relicts of former hard shell magnetite and surface layer lepidocrocite mark earlier growth positions
| Average Tubercle Core, Utility 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ca | Mn | P | Co | Cr | Cu | Pb | Sr | Zn | |
| % | % | % | % | ppm | ppm | ppm | ppm | ppm | ppm | |
| Mains | 59.1 | 0.56 | 0.03 | 0.17 | 29 | 27 | 7 | 96 | 9 | 8 |
| Hydrants | 57.8 | 1.56 | 0.05 | 0.08 | 34 | 40 | 3 | 11 | 21 | 6 |
Ferric-ferrous oxides and carbonates
Ferric-ferrous oxide - Fe3O4 or Fe2O3-FeO. This composition is found in the common iron mineral magnetite, which is ubiquitous in iron corrosion scales. Like hematite, magnetite is a low entropy form and is slow to precipitate at ordinary temperatures. In soils and sediments, there are several types of bacteria that precipitate small magnetite particles that they use to navigate. It is not known whether the magnetite in distribution systems is bacterial, and there do not appear to be good criteria to distinguish bacterial from abiotic magnetite. A common metastable mixed valence phase is "green rust". Rather than a single mineral, this substance appears to be a mixture of Fougèrite, which has the composition Fe2+4Fe3+2(OH)12[CO3]·3H2O with Trébeurdenite which has lower Fe2/Fe3 Fe2+2Fe3+4O2(OH)10CO3·3H2O.
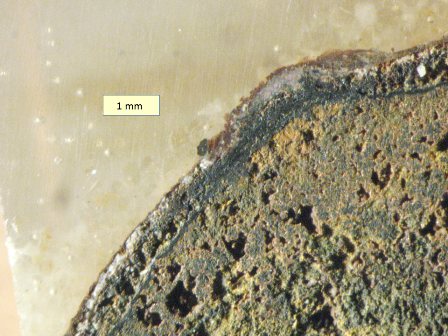
Magnetite in hard shell layer of tubercle in cast iron water main, utility 02. The core of this sample is also magnetite-rich
| Average Hard-shell Layer, Utility 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ca | Mn | P | Co | Cr | Cu | Pb | Sr | Zn | |
| % | % | % | % | ppm | ppm | ppm | ppm | ppm | ppm | |
| Mains | 51.8 | 3.84 | 0.10 | 0.05 | 31 | 17 | 7 | 19 | 31 | 9 |
| Hydrants | 34.8 | 15.8 | 0.07 | 0.04 | 17 | 33 | 10 | 12 | 243 | 9 |
Ferrous Minerals
Ferrous Carbonate - FeCO3. Siderite. This carbonate is fairly common in iron corrosion scales (as is CaCO3). Siderite is well-known from sediments and sedimentary rocks. It typically forms just below the sediment water interface by reaction between iron oxides and carbon from organic matter, as evidenced by light carbon isotopes. Presumably the siderite in corrosion scales forms via a similar route, but there has been little study of it and no isotopic work. The "siderite model" for iron scale development had some popularity at one time (e.g. Sontheimer et al. 1981), but is seldom referred to now and does not appear to have prompted studies of the siderites themselves.
Sontheimer, H., Kolle, W., & Snoeyink, V. L. (1981). The siderite model of the formation of corrosion-resistant scales. Journal-American Water Works Association, 73(11), 572-579.
Back to Iron